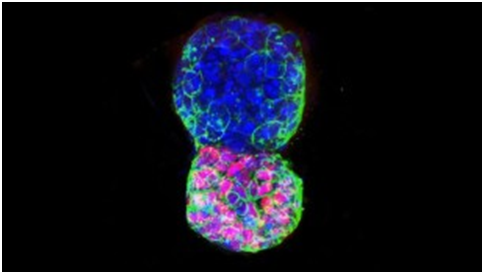
(CNN)Stem cells have the remarkable potential to develop into many different cell types. Now, using mouse stem cells instead of the usual sperm and egg, scientists have created a structure like a blastocyst — an early embryo.
“What we did is, for the first time, we managed to promote the self-organization of stem cells into a very early embryo in a dish — so everything happened in the lab,” said Nicolas Rivron, lead author of the new study and a biologist, engineer and assistant professor at Maastricht University in the Netherlands.
The research was published Wednesday in the international science journal Nature.
To achieve this scientific feat, Rivron and his team combined embryonic stem cells, which have the potential to form a whole embryo, with trophoblast stem cells, which have the potential to form a placenta. Without a placenta, which attaches the embryo to the uterine wall, an embryo cannot develop into a fetus.
“We pulled them together and discovered a cocktail of molecules that triggered them to self-organize into early embryo cells,” Rivron said. He added that, for the first time, these very early embryos were able to implant in the uterus of a mouse when transferred.
Rivron said his research “has applications in many different fields, though we are especially interested in the field of infertility and the field of chronic disease.”
It will allow scientists to learn more about the poorly understood processes of embryo implantation.
FDA sets path for stem cell therapies
“It has been very difficult to understand how [cells] organize in those very early days,” Rivron said. This moment of human development is shrouded in mystery because almost all women don’t even know that they are pregnant at this early stage. Additionally, somewhere between 30% and 60% of embryos fail to implant in humans due to age or unknown factors.
“Human pregnancies are very inefficient, and in the most extreme cases, this is leading to infertility, where people just cannot have a child,” Rivron said.
The study points to other practical applications, he said, including pharmaceutical research.
‘Precious and scarce’
“Because embryos are so precious and scarce, it is almost impossible to test new medicines on them,” Rivron said. “You would need typically huge numbers of embryos in order to test medicines in the proper way. Here, because we are using stem cells, we can generate an infinite number of early embryos.”
Can his process be replicated in other mammals?
“This is the question that is being asked now,” Rivron said. If so, it could be very useful to save endangered species, he said.
Artificial embryo shows early potential for medical therapies, not babies
Magdalena Zernicka-Goetz, a professor of mammalian development and stem cell biology at the University of Cambridge, said the new study “is an excellent beginning.”
It is an important step toward generating “blastocyst-like structures,” said Zernicka-Goetz, who was not involved in the research but has similarly replicated embryonic development using stem cells.
Of the new study, she said, “the resulting structures look like blastocysts and are undertaking some events seen in blastocysts before they mature. However, these blastocyst-like structures do not continue to develop and undertake the events of post-implantation development.”
She believes there are two important goals that neither her own study or the new study has achieved.
“First is to develop structures that contain all three cell layers normally present in the embryo by combining three types of stem cells,” Zernicka-Goetz said. “Second, to show that these structures can truly implant and develop further.”
In short, the next big scientific challenge would be to make “synthetic blastocysts with greater developmental potential,” she said.
Experiments using stem cells are among the most controversial in biological research. Rivron said that although he will create and study mouse embryos using this method, he will not create human embryos.
Noting that such research is not permitted in most countries, he said, “at the moment, it is really a question of: What is going to be the benefit to our society?”
https://edition.cnn.com/2018/05/03/health/embryo-like-structure-stem-cells-study/index.html